Additional information
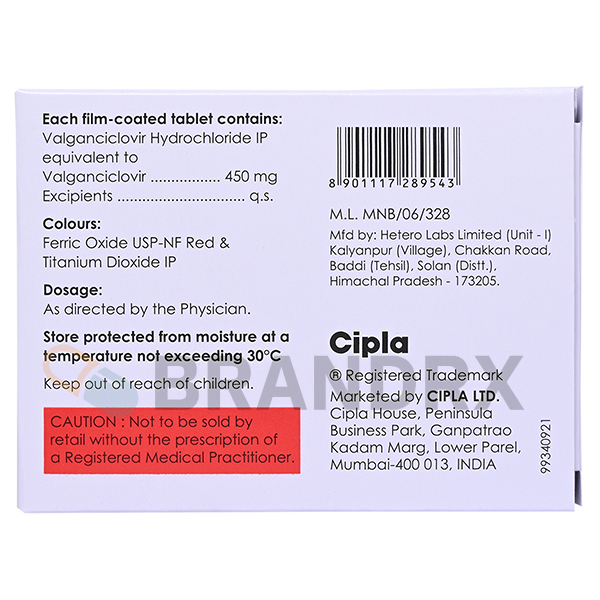
| Active substance | Valganciclovir Hydrochloride |
|---|---|
| Water Retention | Unlikely |
| Hepatotoxicity | Low risk but can occur |
| Lab Test | Monitoring of complete blood count and renal function tests are recommended |
| Strength | 450mg |
| Also known as | Valcyte |
| Blood pressure | No direct effects known |
| Trade name | Valcyte |
| Storage conditions | Store at room temperature between 15В°C and 30В°C (59В°F and 86В°F) |
| Chemical name | Valganciclovir hydrochloride |
| Formula | C14H22N6O5 |
| Substance class | Antiviral medication |
| Main action | Inhibits the replication of viral DNA |
| Half-life | 4 hours |
| Dosage (medical) | Typically 900 mg taken orally twice daily for induction therapy, and 900 mg once daily for maintenance therapy |
| Dosage (sports) | Not applicable |
| Effects | Reduction in viral load and improvement in symptoms associated with cytomegalovirus (CMV) infections |
| Side effects | Diarrhea, neutropenia, fever, anemia, leukopenia, and potential retinal detachment |
| Use in sports | None |
| Manufacturer | Cipla Ltd. |
| Packing | 4 tabs/blister |


Reviews
There are no reviews yet.